Introduction to Liquid Dielectrics
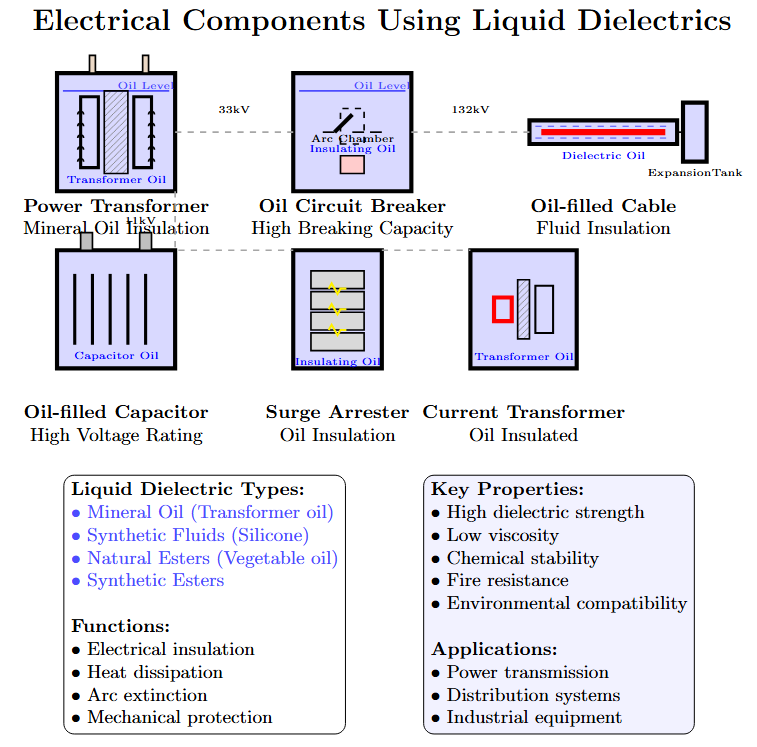
Liquid Dielectrics: Applications
-
Used in transformers, circuit breakers, high-voltage cables, and capacitors.
-
Functions:
-
Insulation between live and grounded parts.
-
Heat dissipation in transformers.
-
Arc quenching in circuit breakers.
-
-
Common liquids: Petroleum oils, synthetic hydrocarbons, silicone oils, fluorinated hydrocarbons.
Properties of Liquid Dielectrics
Key Properties of Liquid Dielectrics
-
Primary Properties:
-
Dielectric strength
-
Dielectric constant (relative permittivity)
-
Electrical conductivity
-
-
Other Properties:
-
Viscosity, thermal stability, specific gravity, flash point.
-
-
Dielectric strength affected by:
-
Fine water droplets (0.01% water reduces strength to 20% of dry oil).
-
Fibrous impurities (sharply reduce strength).
-
Temperature and pressure variations.
-
Selection of Liquid Dielectrics
-
Primary Consideration: Chemical stability.
-
Other Factors:
-
Cost
-
Space savings
-
Environmental susceptibility
-
Fire safety (flash point)
-
Biodegradability
-
-
Impact: Liquid dielectrics enable compact equipment (e.g., 765kV transformers infeasible with air insulation).
Dielectric Properties of Common Liquids
| Property | Transformer Oil | Capacitor Oil | Cable Oil | Silicone Oil |
|---|---|---|---|---|
| Relative permittivity (50Hz) | 2.2–2.3 | 2.1 | 2.3–2.6 | 2.7–3.0 |
| Breakdown strength (20\(^{\circ}C\), 2.5mm, 1min) | 12kVmm−1 | 18kVmm−1 | 25kVmm−1 | 35kVmm−1 |
| Tan \(\delta\) (50Hz) | 1×10−3 | 2.5×10−4 | 2×10−3 | 1×10−3 |
| Tan \(\delta\) (1kHz) | 5×10−4 | 1×10−4 | 1×10−4 | 1×10−4 |
| Resistivity (\(\Omega \mathrm{cm}\)) | 1×1012–1×1013 | 1×1013–1×1014 | 1×1012–1×1013 | 2.5×1014 |
| Max. permissible water (ppm) | 50 | 50 | 50 | <40 |
| Acid value (mg/g) KOH | 0.2 | 0.03 | 0.2 | 0.05 |
| Saponification (mg/g) KOH | 0.01 | 0.01 | 0.01 | <0.01 |
| Specific gravity (\(20~^{\circ}C\)) | 0.89 | 0.89 | 0.93 | 1.0–1.1 |
Pure vs. Commercial Liquids
Pure Liquids:
-
Chemically pure, structurally simple
-
Minimal impurities (1 per billion)
-
Consistent behavior
-
Higher dielectric strength
-
Expensive and difficult to maintain
Commercial Liquids
-
Chemically impure, complex organic mixtures
-
Erratic behavior; no two samples behave identically
-
Lower dielectric strength
-
Cost-effective
-
Practical for industrial use

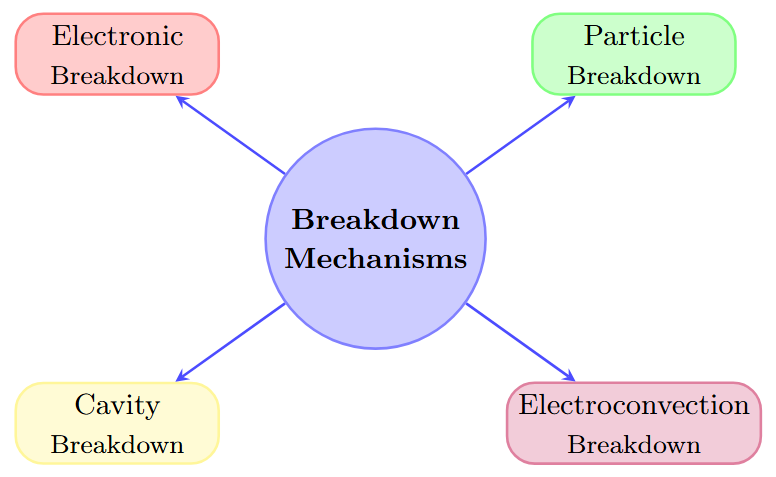
Breakdown Mechanisms
Breakdown Mechanisms in Liquids
-
Breakdown theory less understood compared to gases or solids.
-
Four main breakdown mechanisms:
-
Electronic breakdown (avalanche ionization)
-
Suspended particle breakdown
-
Cavity breakdown (gas bubble formation)
-
Electroconvection breakdown
-
Breakdown Mechanism: Electronic Model
-
Based on avalanche ionization from electron collisions.
-
Electrons ejected from cathode via:
-
Field emission.
-
Field-enhanced thermionic effect (Schottky’s effect).
-
-
Applies to highly pure liquids, not commercial ones.
-
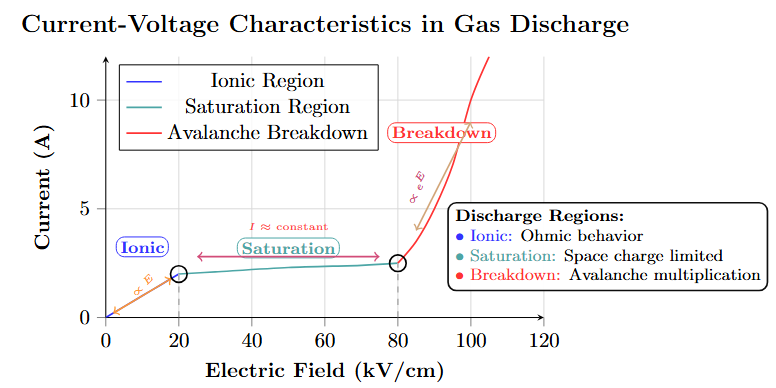
Conduction behavior:
-
Low field (1kV/cm): Ionic, linear increase.
-
Moderate field: Saturation.
-
High field (100kV/cm): Rapid increase, leading to breakdown.
-
Current vs. Electric Field Characteristics
Three Regions:
-
Linear region (ionic)
-
Saturation region
-
Rapid increase region
Electronic Breakdown Mechanism
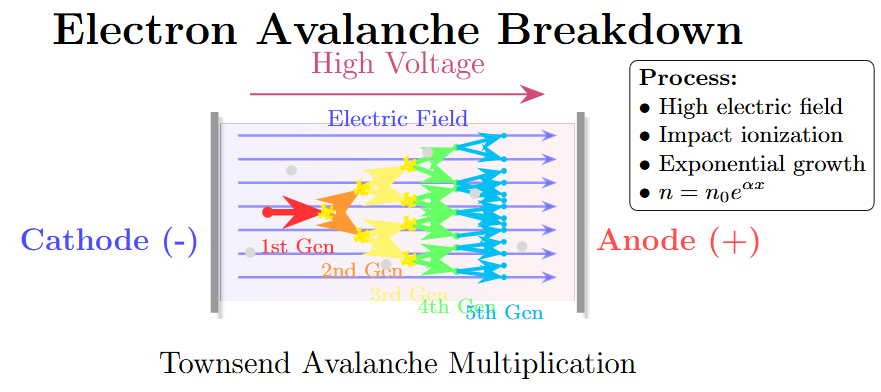
Process Description
-
Electrons gain energy from an applied electric field.
-
Some electrons gain more energy than they lose in collisions, leading to acceleration.
-
Accelerated electrons gain sufficient energy to ionize molecules, initiating an avalanche.
Threshold Condition
The avalanche begins when the energy gained equals the energy lost during ionization:
-
\(\lambda\): mean free path
-
\(h \nu\): ionization energy
-
\(C\): constant
Dielectric Strengths of Pure Liquids
| Liquid | Strength (MV/cm) |
|---|---|
| Benzene | 1.1 |
| Transformer Oil | 1.0–4.0 |
| Hexane | 1.1–1.3 |
| Nitrogen | 1.6–1.88 |
| Oxygen | 2.4 |
| Silicone Oil | 1.0–1.2 |
Observation
The electronic theory predicts dielectric strength well but overestimates formative time lags compared to observations.
Suspended Solid Particle Mechanism
Introduction
Commercial liquids contain solid impurities (fibers or dispersed particles) with permittivity \(\epsilon_1\) different from the liquid’s \(\epsilon_2\).
Force on Particles
A spherical particle of radius \(r\) in an electric field \(E\) experiences a force:
-
Directed toward higher field stress if \(\epsilon_1 > \epsilon_2\).
-
Directed toward lower field stress if \(\epsilon_1 < \epsilon_2\).
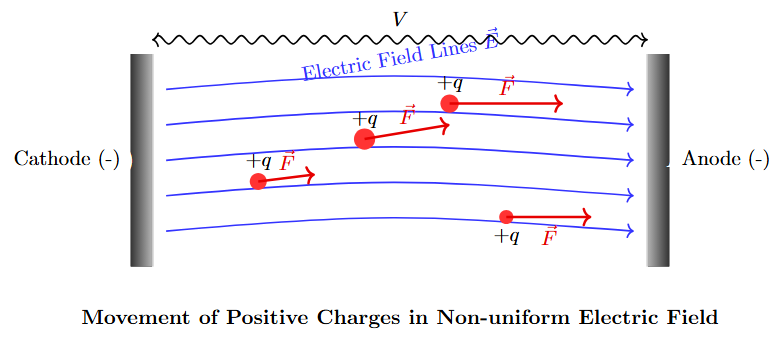
Particle Force in Limiting Cases
High Permittivity Limit
If \(\epsilon_1 \to \infty\):
Behavior in Uniform Field
In a uniform field (e.g., small sphere gap), \(\frac{dE}{dx} \to 0\), so \(F = 0\). Particles remain in equilibrium and are dragged into the uniform field region.
Particle-Induced Breakdown
Mechanism:
-
Particles with \(\epsilon_1 > \epsilon_2\) cause flux concentration at their surface.
-
Large particles align to form a bridge across the gap, increasing the field.
-
If the field exceeds the dielectric strength, breakdown occurs.
-
Insufficient particles cause local field enhancement, leading to gas bubble formation and eventual breakdown.
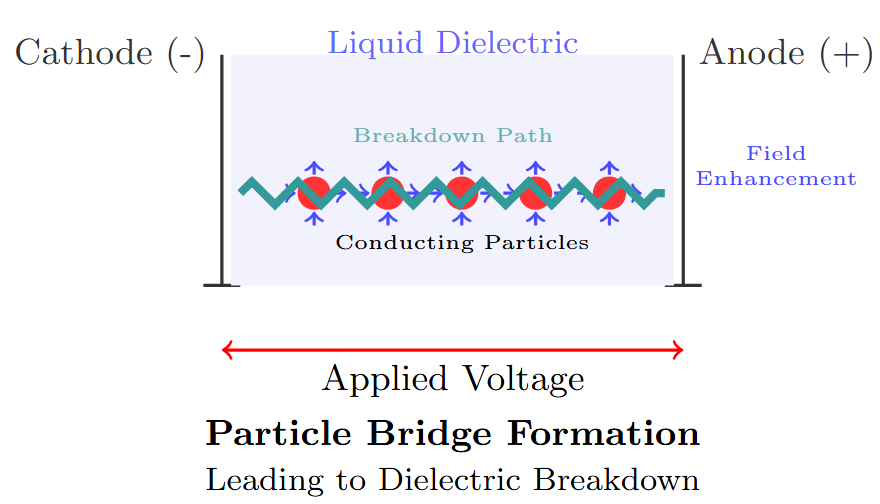
Particle Motion and Forces
Viscous Force
The viscous force opposing particle motion (Stokes’ relation):
Equating Forces
Balancing electrical and viscous forces:
Key Insight
Particle velocity depends on:
-
Particle size (\(r^2\) dependence)
-
Field gradient (\(dE/dx\))
-
Liquid viscosity (\(\eta\))
Including Diffusion Effects
Drift Velocity Due to Diffusion
The drift velocity due to diffusion (Stokes-Einstein relation):
Equating Velocities
Equating electrical and diffusion velocities:
Cavity Breakdown
Cavity Breakdown: Overview
-
Dielectric strength of liquids depends on hydrostatic pressure.
-
Higher pressure increases electric strength, suggesting phase change involvement.
-
Smaller liquid head increases chances of partially ionized gases escaping, leading to breakdown.
-
Vapour bubble formation is key to the breakdown process.
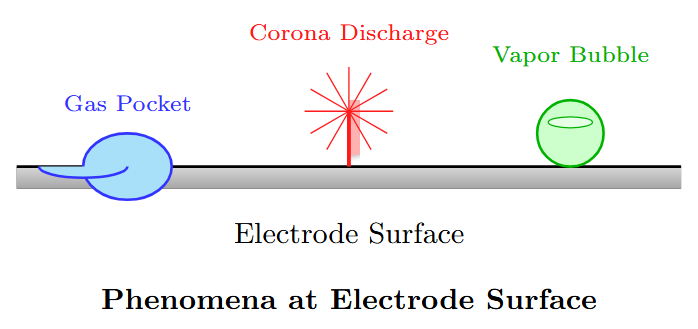
Processes Leading to Bubble Formation
-
Gas pockets on electrode surfaces.
-
Point charge concentration at irregular electrode surfaces causing corona discharge, vaporizing liquid.
-
Changes in temperature and pressure.
-
Dissociation of products by electron collisions producing gaseous products.
Electric Field in Gas Bubble
-
Field in a gas bubble immersed in a liquid of permittivity \(\epsilon_2\):
\[E_b = \frac{3 E_0}{\epsilon_2 + 2}\]where \(E_0\) is the field in the liquid without the bubble. -
Bubble elongates under \(E_0\), maintaining constant volume.
-
When \(E_b\) equals gaseous ionization field, discharge occurs, potentially causing liquid decomposition and breakdown.
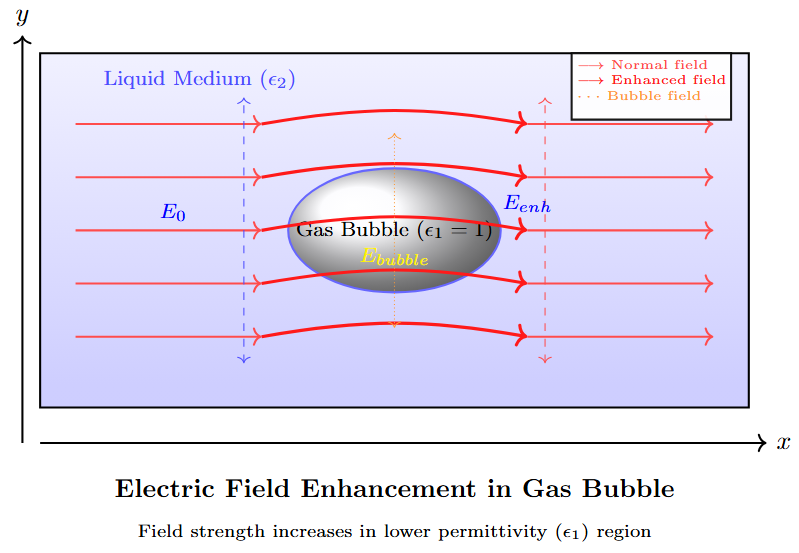
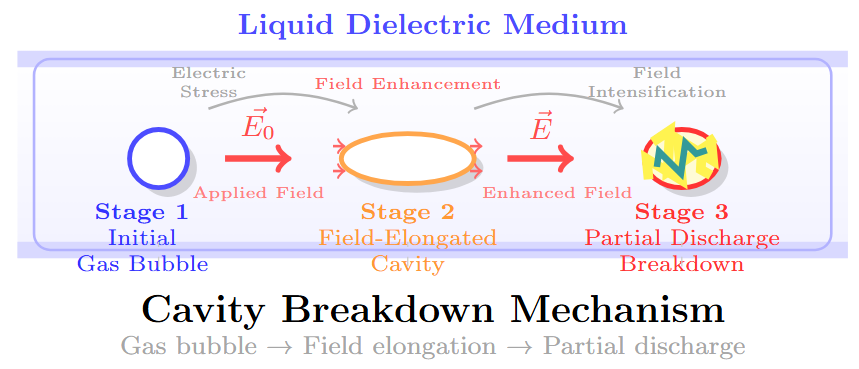
Bubble Elongation and Breakdown
Bubble Dynamics
-
Bubble elongates in field direction while maintaining constant volume.
-
Elongation increases field enhancement factor.
-
Critical field reached when discharge initiates in the bubble.
Breakdown Criterion
Breakdown occurs when:
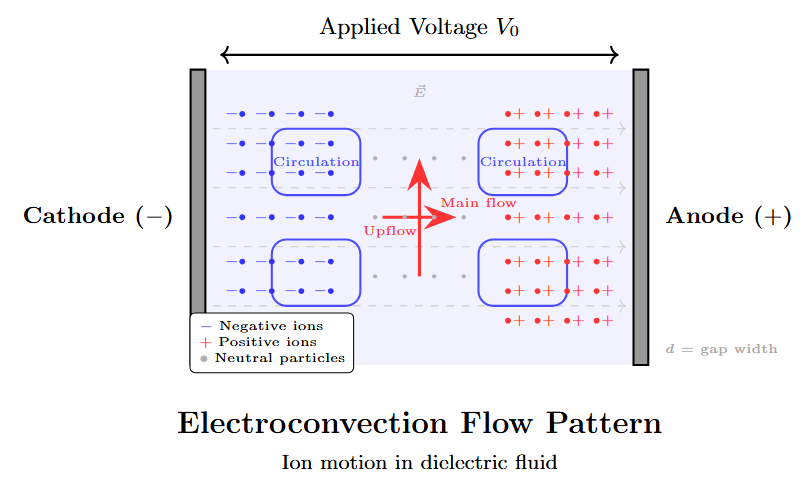
Electroconvection Breakdown
Electroconvection Mechanism
-
Occurs in pure liquids with low electrical conductivity.
-
Current flow creates space charge, leading to mechanical forces.
-
Mechanical forces cause fluid motion (electroconvection).
-
Fluid motion brings impurities to high-field regions, causing breakdown.
Electroconvection Process
-
Charge injection from electrodes
-
Space charge formation
-
Coulomb forces on charge carriers
-
Fluid motion and turbulence
-
Impurity concentration in high-field regions
-
Breakdown initiation
Electroconvection Flow Pattern
Factors Affecting Electroconvection
Promoting Factors:
-
High electric field
-
Low viscosity
-
Charge injection efficiency
-
Electrode geometry
-
Temperature increase
Inhibiting Factors:
-
High viscosity
-
Uniform field distribution
-
Low charge mobility
-
Hydrostatic pressure
-
Temperature control
Practical Considerations
Breakdown Prevention Strategies
-
Liquid Purification:
-
Remove water content below 50ppm
-
Filter out solid particles
-
Degassing to remove dissolved gases
-
-
Electrode Design:
-
Smooth, rounded electrodes to avoid field concentration
-
Proper spacing and geometry
-
Surface treatment to minimize roughness
-
-
System Design:
-
Pressurized systems to prevent bubble formation
-
Temperature control
-
Circulation and filtration systems
-
Testing and Monitoring
Routine Tests:
-
Dielectric strength test
-
Water content analysis
-
Dissolved gas analysis (DGA)
-
Acidity and neutralization tests
-
Interfacial tension measurement
Monitoring Parameters:
-
Partial discharge activity
-
Temperature variations
-
Pressure changes
-
Current leakage
-
Color and appearance
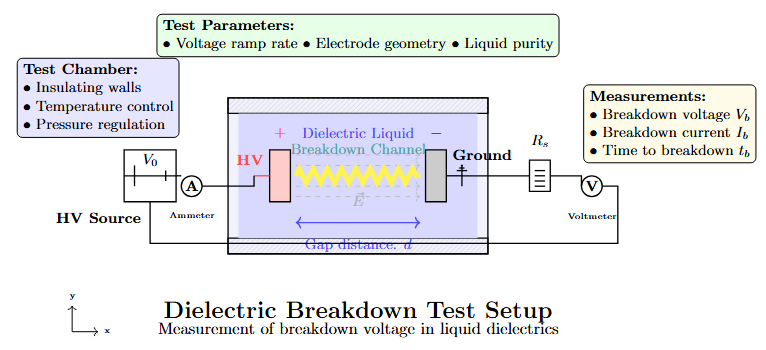
Conclusion
Summary
-
Liquid dielectrics are essential for high-voltage equipment insulation.
-
Four main breakdown mechanisms exist:
-
Electronic breakdown (pure liquids)
-
Suspended particle breakdown (commercial liquids)
-
Cavity breakdown (gas bubble formation)
-
Electroconvection breakdown (fluid motion)
-
-
Practical breakdown is usually a combination of these mechanisms.
-
Proper maintenance, purification, and monitoring are crucial for reliable operation.
-
Understanding breakdown mechanisms helps in designing better insulation systems.
Future Developments
-
Development of biodegradable dielectric liquids
-
Nano-enhanced dielectric fluids
-
Advanced diagnostic techniques
-
Computational modeling of breakdown processes
-
Smart monitoring systems with AI integration
